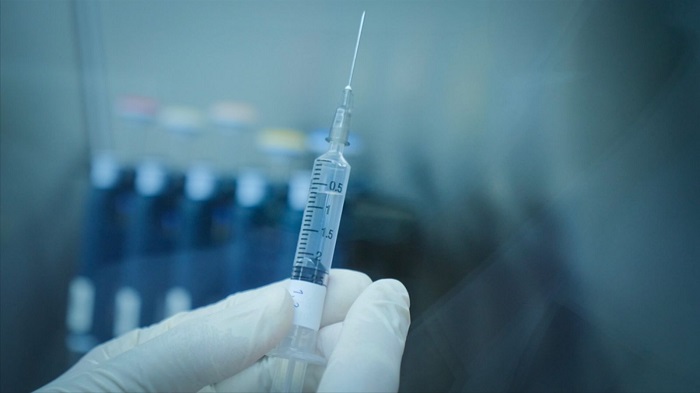
China has commissioned a locally-produced Coronavirus Vaccine.
The vaccine is subject to assessment by the World Health Organization (WHO) before it can be made available for use around the world.
Speaking at a virtual press conference,
Socorro Escalate, the coordinator for essential medicines and health technologies in the Western Pacific region for WHO said China had held preliminary discussions with WHO to have its vaccines included in a list for emergency use.
“The WHO’s emergency use listing procedure allows unlicensed vaccines and treatments to be assessed to expedite their availability in public health emergencies.”
“This helps assist the WHO’s member states and UN procurement agencies to determine the acceptability of the vaccines.”
“Potentially through this emergency use listing the quality and safety of these vaccines and efficacy could be assessed and then this could be made available for our licensees,” according to Escalante.
Meanwhile, hundreds of thousands of essential workers and other groups considered at high risk in China have been given locally-developed vaccines even as clinical trials had not been fully completed which have raised serious safety concerns among health experts.
By Melvin Tarlue